About the CAVA Project
In England and Wales, 8 out of every 1,000 patients are likely to consult their GP complaining of dizziness every year.
Dizziness is the most common reason for a doctor to visit a patient over the age of 75 years old, and one-third of adults over the age of 65 years experiences at least one fall each year. This is particularly important as the cost of falls to the NHS and social services in people over 60 years old in 1999 was £981 million.
However, we are currently significantly restricted with respect to what specialist tests are available for assessing patients with dizziness, because there are no tests available to evaluate a patient during an actual dizziness attack in the community. This leads to repeated and expensive hospital visits before a diagnosis can be made. The US National Institutes of Health reports that the average number of doctors that a patient with dizziness visits before receiving a correct diagnosis is 4.5. The CAVA project aims to overcome these limitations of the current tests by developing a dizziness monitor from which an accurate diagnosis can be made.
Long before the MRC grant was awarded, initial proof of concept prototypes were developed by Mr John Phillips and Professor Stephen Cox, at the University of East Anglia. The prototypes proved that the concept was viable and the results from that preliminary work formed the basis of a successful MRC grant application.
Continuing from that early work, the aim of this grant is to develop a bespoke device that is lightweight, durable, and able to be worn day and night. The project also requires the development of specialist computer software to analyse the data produced by the CAVA device. Once fully developed and tested in clinical studies, it is hoped that our device will be made available at the point of initial referral to a doctor or nurse to avoid delay in diagnosis and ensure cost-effective use of appropriate resources.
Continuing from that early work, the aim of the project is to develop a bespoke device that is lightweight, durable, that can be worn day and night, and that will record data from which diagnosis of dizziness conditions may be made. Such diagnosis requires the development of advanced computer software to analyse the CAVA data.
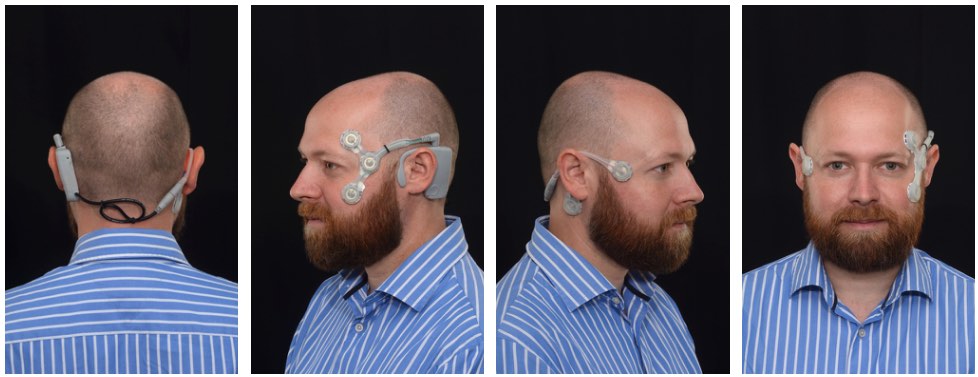
Working with a design agency (Wright Design Limited), we have successfully designed, developed and produced a fully functional medical prototype device for appraising dizziness. The device comprises a logging module which sits behind the left ear, and two single-use electrode mounts which attach to each side of the face. The device is easy to apply and requires minimal user intervention during operation.

We then conducted two sets of trials with the device. The first was using 18 healthy volunteers who had a dizziness symptom artificially induced in them, and it confirmed that subjects had no problems in wearing the CAVA device 23/7 for 30 days, and that the software we had developed could detect the presence of a dizziness signal with very high accuracy (see the first publication on https://www.uea.ac.uk/groups-and-centres/cava-project/publications for details). The second trial was conducted on patients suffering from inner-ear conditions that cause dizziness, and the CAVA device succeeded in capturing data corresponding to episodes of vertigo and other conditions that had never been observed before (see publication no 9 on https://www.uea.ac.uk/groups-and-centres/cava-project/publications).
With funding from the National Institute for Health Research, the CAVA project is now conducting a large-scale trial at ten hospital centres around the UK. The goal of this trial will be to collect much more clinical data from patients with dizziness symptoms which will be used to enable diagnosis of three common conditions. In parallel with this, the device and its accompanying software also be developed to make it commercially available to clinicians worldwide.
Related Links
Nature: Scientific Reports publication
Dizziness Trial on ClinicalTrials.gov
Healthy Volunteer Trial on ClinicalTrials.gov
Contact Us
T: 01603 593054
CAVA Office (BIO 2.19)
School of Computing Sciences
University of East Anglia
Norwich Research Park
Norwich
NR4 7TJ


This project was funded by the Medical Research Council and the NIHR.

)