)

“We can grow human heart muscle in a dish now,” explains Dr James Smith, a Group Leader at Norwich Medical School at UEA. “I could take you over to the Research Park and show you human heart tissue in that we have made from stem cells.”
Since 2019, Smith has headed up a lab at UEA using stem cells to investigate heart disease - and he’s just secured £1m of research funding from the British Heart Foundation, to be split between his team at UEA, and researchers at the University of Nottingham and the University of Liverpool.
This means he can continue his cutting-edge work on hypertrophic cardiomyopathy: a genetic condition that means the walls of heart are thicker than usual, leading to difficulties in heart function, and even death.
A sheet of lab-grown, stem cell-derived heart tissue produced in Dr Smith’s laboratory at UEA. Image credit: James Smith
Dr Smith is using stem cells to generate human heart tissue in a dish, where it spontaneously beats in a manner similar to patient heart tissue (see video above). Using cutting-edge gene editing techniques, he and his team are learning more about how specific gene mutations alter the signaling pathways in the heart.
“We want to improve our understanding to discover treatments to improve the lives of patients with heart disease, some of whom could be at risk of sudden cardiac death,” he explains. “We’re getting insights into heart disease that could lead to the development of new treatments.”
It’s a rapidly changing area of research, he continues, largely thanks to stem cells opening up a whole new world of biological understanding.
“So now, if I want to learn about why you have that heart disease, all I need to figure it out is a bit of your blood or a bit of your skin.”
“Let's say you have a heart disease and I want to study why your heart is struggling and why you have that disease,” Dr Smith says. “It's going be very difficult for me to get hold of some of your heart tissue. You're not going to be able to say: ‘OK, just cut open my chest and I'll give you some heart tissue.’”
And that, he explains, is where pluripotent stem cells have changed things. These are cells that are in an early state of development and can mature into all kinds of different cells, like bone, or brain, or, indeed, heart. It was previously assumed that mature cells could not return to this state, until research from Japanese scientist Shinya Yamanaka confirmed that genetic editing can switch cells back (for which he was awarded the Nobel prize in 2012).
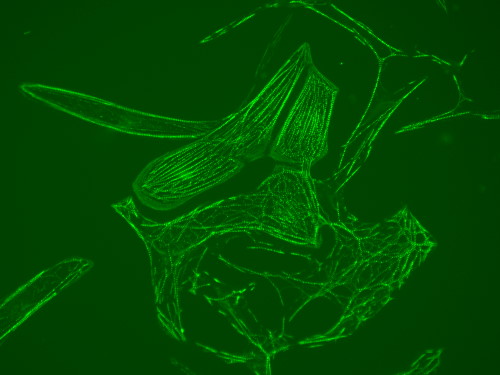
Cardiomyocytes (heart cells) made from stem cells. The internal structure (sarcomere) that allows the cells to beat, is labelled using a green fluorescent protein. Image credit: James Smith
“That's the beauty of pluripotent stem cells,” Dr Smith explains, “they’re almost the same as embryonic ones, but without going near an embryo. Often people have heard of stem cells, but they associate it with the ethical controversy of working with embryos.”
Instead, cells that have become specialised can now be returned to their earlier, stem-cell state and then manipulated to create other cells. In this case, heart cells.
“So now, if I want to learn about why you have that heart disease, all I need to figure it out is a bit of your blood or a bit of your skin,” Dr Smith continues. “I can reprogram that back into a pluripotent stem cell and that can become anything, so I can turn it into heart cells. And they won't be any old heart cells, they'll be your heart cells.”
“It's literally in the last 10 years that this has taken off, and it's getting better every year, it's exploding.”
“It's literally in the last 10 years that this has taken off, and it's getting better every year, it's exploding,” he says. “Making pluripotent stem cells was a technique that first happened in 2005. And that's around when I started university. You had to be on the cutting edge to work with these. I was lucky that I got a post-doc position at [the University of] Nottingham and it was a good lab. So I learned how to do this early on in the technology.
So what now for this potentially revolutionary technique? For Dr Smith and his lab at UEA, it’s all about disease modelling: finding out “why you have that disease and what is actually going wrong when you have those genetic mutations, so that maybe we'll find drugs and therapies that will help you”. And using pluripotent stem cells means that this work can take place at a hitherto unimaginable rate.
“Imagine doing a clinical trial, where you want to see which drugs can help people. Ethically, you obviously can't go into a hospital and give heart patients thousands of different drugs and see which ones work. But now we can take cells from all of those people, and expose those heart cells in a dish to thousands of different drugs and find out which ones work.”
And that’s where patients with this genetic mutation at the Norfolk and Norwich University Hospital are also contributing to this groundbreaking research, Smith explains. “Patients there [with hypertrophic cardiomyopathy] are now given the option to consent to give us blood, and then we can study it to see if our findings from the stem cell models correlate with what's happening in their blood,” he explains. “As well as the doctors looking at their blood to see how they're doing, they are also allowing our scientists at UEA to look at their blood and see if it helps them to understand this disease better.”