Healthy Volunteer Trial
The CAVA Healthy Volunteer Trial ran in the latter part on 2018. The purpose of this trial was to evaluate the CAVA device's capability to capture long-term eye movement data for the purpose of retrospectively detecting dizziness. As the trial participants were healthy individuals who do not experience episodes of dizziness, it was necessary to simulate the characteristic dizziness eye movements (nystagmus - see figure below) by way of an optokinetic stimulus viewed on a VR headset.
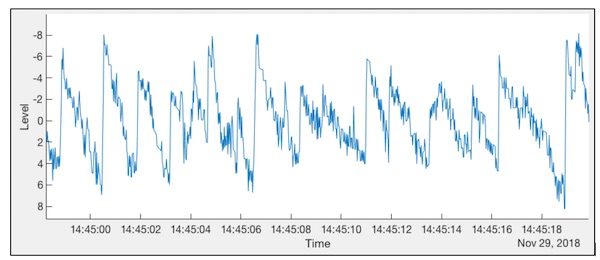
Each participants was given a list of days on which they had to induce nystagmus (typically eight days in the thirty day trial). The task of the project’s research associate (Jacob Newman, JN) was to use internally developed computer algorithms to detect the presence or absence of this 30s of nystagmus in each day’s worth of trial data. The co-investigator (Prof. Stephen Cox, SC) and JN developed several computer algorithms in parallel in order to find the best approach to the problem. These approaches included various machine learning techniques applied to both the time domain and frequency domain signals. The values of sensitivity and specificity attained by the algorithm when applied to the trial data demonstrated that we could confidently identify a minimum duration of thirty seconds of optokinetic-induced nystagmus from data captured by the CAVA device.

As well as capturing good quality eye movement data, the device was found to be reliable, with very few technical issues reported during a total deployment time of more than 400 days. The device was also praised by the trial participants. Participants were asked to fill in a short questionnaire, the purpose of which was to gather feedback on the device and on their experiences of the trial.
In terms of device usability, all participants questioned found the device to be both easy to apply and to remove from the face. The majority of participants found it easy to sleep whilst wearing the device and they generally found the device to be comfortable to wear. Finally, with respect to the social implications of wearing the device, all participants reported that the device did not interfere greatly with their normal daily activities, and the majority expressed that they did not feel self-conscious whilst wearing the device.
The results from the first trial have shown both the device and algorithms developed to be fit for purpose. In several areas, the results have exceeded our original expectations, including the completion of several strands of research that we did not intend to complete at this early stage. Based on these promising results, in early 2019 we will start the process of preparing our applications to the relevant regulatory bodies in order to receive permission to start a second trial on individuals with dizziness.

Related Links
Nature: Scientific Reports publication
Dizziness Trial on ClinicalTrials.gov
Healthy Volunteer Trial on ClinicalTrials.gov


This project was funded by the Medical Research Council and the NIHR.

)