Background
To deliver healthy infants, women with diabetes are advised to aim for near normal blood glucose levels (3.9-7.8 mmol/L). The importance of avoiding hyperglycaemia to reduce preterm delivery, neonatal morbidity and large for gestational age (infant birth weight >90th percentile) is well recognised.
Studies in pregnant women with and without diabetes suggest that for normal fetal growth mean blood glucose of 5.3 mmol/L is required throughout the second and third trimesters. Using Continuous Glucose Monitoring (CGM) we have shown that pregnant women with type 1 diabetes spend an average 12 hrs/day or 50% time within the NICE recommended glucose target levels of 3.9-7.8 mmol/L, with mean CGM glucose levels of 7.1 mmol/L and 6.6 mmol/L during the second and third trimesters respectively. There is an urgent unmet need for better tools to improve glucose control and maternal infant health outcomes in type 1 diabetes pregnancy.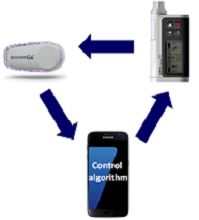
The three components of the closed-loop system are an insulin pump, a continuous glucose monitor (CGM) and a computer-based model predictive control (MPC) algorithm to compute information from the CGM into a recommended insulin dose. Closed-loop systems are designed to deliver insulin in response to CGM glucose levels and may help to bridge the gap between our expectations of tight glucose control and what is currently achievable using standard insulin pumps and injections.
Previous, non-randomized feasibility studies suggest that the automated closed-loop system can cope not only under steady-state glucose conditions but also under more challenging circumstances of changing insulin resistance during advanced pregnancy encompassing antenatal steroids, labour, delivery and the immediate post-partum period.
The AiDAPT trial focuses on determining the definitive proof of clinical efficacy in women using automated closed-loop for approximately 28 weeks duration (10-38 weeks) throughout pregnancy in real-life NHS antenatal care settings. It will also aim to understand more about women’s and health care professionals’ experiences of using automated insulin delivery in type 1 diabetes pregnancy and to provide estimates of its cost-effectiveness and cost-utility.
Trial Design
AiDAPT is an open-label, multi-centre, randomized, two-arm parallel group trial comparing automated closed-loop and standard insulin delivery. Women fulfilling the eligibility criteria will be randomized to automated insulin delivery (AiD) or to continue standard patient-directed insulin delivery (CSII or MDI) with CGM.
Objectives
The primary efficacy endpoint is the percentage time spent with glucose levels within the NICE target range of 3.9-7.8 mmol/L, as recorded by CGM in both groups.
The safety aspects of automated insulin delivery will be assessed, by collecting data on episodes of severe hypoclycaemia, diabetic ketoacidosis and adverse device effects.
Participant’s perception of automated insulin delivery will be assessed using several validated questionnaires. Health care professional’s experiences of using the closed-loop system in NHS settings will also be assessed.
Study population
124 pregnant women between 18 and 45 years of age with Type 1 diabetes (T1D) of at least 12 months’ duration on standard insulin delivery (CSII or MDI) will be recruited through outpatient antenatal diabetes clinics.
Participants must be willing to use the study devices, have email access and be able to give Informed consent
Potential participants with any physical or psychological disease which may interfere with conduct and interpretation of the study, and participants using contraindicated medication cannot be included in the study.
When is the study starting?
The study opened to recruitment in October 2019.
Study sponsor
AiDAPT is sponsored by Norfolk and Norwich University Hospitals NHS Foundation Trust and co-ordinated by Norwich Clinical Trials Unit.

)