Medicines use in care homes has been shown to be suboptimal and it has been suggested that to address this, one person should assume overall responsibility for medicines management in a care home.
CHIPPS - The Care Homes Independent Pharmacist Prescribing Study (CHIPPS), is a 5-year, NIHR-funded research programme, which proposed that a suitable model for appropriate medicines management in care homes was a Pharmacist Independent Prescriber, who would assume responsibility for repeat prescriptions’ monitoring and authorising and overall management of medicines in the care home.
The PIP would use personal pharmaceutical care plans (PCPs) to communicate prescribing decisions and plans between members of the care team.
CHIPPS developed this innovative new model of care and then tested it in a feasibility study before completing a Randomised Controlled Trial. The programme of work consisted of the following six work packages:
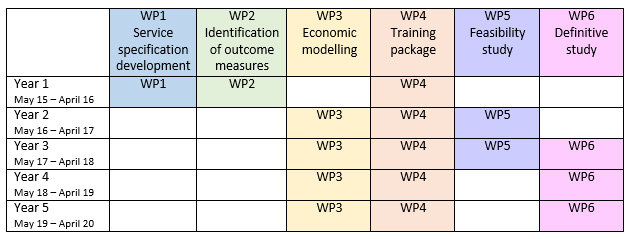
Work package 1 Service specification development (months 1-12)
- Phase 1- Literature review update (completed)
- Phase 2- Stakeholder meetings (completed)
- Phase 3- Service-specification synthesis (completed)
Work package 2 Identification of outcome measures (months 1-15)
- Literature review (completed)
- Consensus process (completed)
Management committee review (completed) - Dissemination (completed))
Work package 3 Economic modelling (months 13-60)
- Identification of costs and development of collection instruments (completed)
- Test feasibility study tools (completed)
- Review and finalise data collection process (completed)
- Develop economic models and dissemination (completed)
Work package 4 Training package (months 1-57)
- Training needs analysis (focus groups/interviews completed; analysis and data synthesis in progress) (completed)
- Development of training package (completed)
- Delivery of prototype training package (completed)
- Review and redesign of training package (completed)
- Delivery of revised training package (completed)
- Final review of training package and dissemination (completed)
Work package 5 Feasibility study (months 13-30)
- Ethical approval (completed)
- Identify medical practices and homes (completed)
- Recruit residents (completed)
- Feasibility study delivery (completed)
- Baseline and follow-up data collection (completed)
- Data analysis and review (completed)
- Stage report and dissemination (completed)
Work package 6 Definitive study (months 25-60)
- Protocol revision (completed)
- Ethical approval (completed)
- Identify medical practices and homes (completed)
- Recruit residents (completed)
- Internal pilot (completed)
- Progress review and report (completed)
- Main trial (completed)
- Data collection (completed)
- Qualitative data collection and analysis (completed)
- Data analysis and dissemination (completed)

)