1. To introduce basic concepts of organic synthesis:
Retrosynthesis – thinking backwards from relatively complex molecules to simpler ones – the disconnection approach.
2. To classify and extend the main carbon-carbon bond forming reactions (CCBFR) introduced in CHE1C1Y.
3. To illustrate the importance of organic synthesis with real examples.
Organic Synthesis
Introduction
Why bother?!
Ca. 7 million organic compounds known – most have been made by synthesis rather than isolated from nature.
Reasons for Synthesising Organic Compounds:
a) Proof of structure of a natural compound by ‘putting it together’ from simpler molecules.
b) To prepare compounds that are useful to mankind e.g. pharmaceuticals, polymers, dyes etc.
c) To prepare specific compounds to study reaction mechanisms or biological metabolism (e.g. labelled compounds).
d) For the intellectual challenge – new problems demand new solutions and can lead to the development of NEW CHEMISTRY, reagents etc.
When faced with the challenge of preparing a specific organic compound ho do we go about it?
This is the art of synthesis!
e.g. How might we attempt to make Z jasmone – an important constituent of many perfumes?
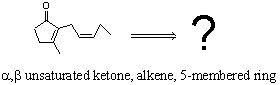
In fact one synthesis uses the following as carbon sources:
It is not clear from this however, how the chemistry
might be done! Therefore just being given the starting materials is
not sufficient to help plan a synthesis.
Note the importance of CCBFR.
We need a logical planning method.
Retrosynthetic Analysis (The Disconnection Approach)
Originated by E.J. Corey (Nobel Prize 1990) p169 – 172
p259 – 260
p354 – 359
Target Molecule (TM) – the compound we wish to prepare e.g.
Retrosynthetic Analysis – the process of WORKING BACKWARDS from the TM in order to devise a suitable synthetic route (or routes) on paper.
Note:
Multiple steps are required so this needs to be repeated.
After writing possible routes we would need to evaluate each one before deciding which to follow.
Readily Available Starting Materials (RASM) – cheap, commercially available compounds.
Disconnection – a paper operation involving an imagined cleavage of a bond (yielding ‘synthons’) to suggest a method and possible SM’s for making the bond, ultimately leading to possible SM’s for the overall synthesis.
Note: There must be a good chemical reaction corresponding to the reverse of the disconnections.
Synthon – an idealised fragment, usually a cation or anion, resulting from a disconnection.
Usually synthons don’t exist as such, but help in the correct choice of reagent.
In our example:
Synthetic Equivalent – the actual compounds used to function as synthons.
Functional Group Interconversion (FGI) – the process of writing one functional group for another to help synthetic planning and to help disconnection. Note, there must be a good reaction in the reverse (forward!) direction.
e.g.
Alternative synthesis of
Many ways to make alcohols (e.g. via Grignard reagents) - suggests alternative synthesis to Friedel Crafts.
In planning a synthetic strategy, apart from devising a means of constructing the carbon skeleton with the required functionality as above, there are other subtle factors, which we must address.
(Good summary p169 – 172, 259 – 260, 354 – 359)
We will illustrate this approach with examples, starting with synthesis of benzene derivatives. Starting point is usually fairly obvious – simple benzene derivatives or perhaps benzene itself.
Reactions are usually straightforward (SEAr) and you will have met most of them before. Synthesis is simplified because the nature of the starting materials is usually clear. Thus, most reactions correspond to the following disconnection:
1st decision – which bond to disconnect first!
However, we can carry out monobromination on the N-acyl derivative of the amine:
then we can remove the protecting group (HO-/H2O) to give the required product.
So formally:
Is there an alternative route? Try a different FGI!
Example 2
Example 3
Guidelines for designing a synthesis
Clearly you will need a good knowledge of your basic chemistry and an appreciation of reaction mechanisms, directing effects etc.
With Aromatic systems the SM’s are usually fairly obvious. Usually benzene or a benzene derivative such as toluene, phenol etc. bond to be disconnected is almost always the bond joining the aromatic ring to the rest of the molecule.
Also FGI’s often correspond to some simple types of reaction
e.g. reduction (NO2 to NH2), oxidation (CH3
to COOH), diazonium chemistry (NH2![]() N2+
N2+ ![]() Ar-X).
Ar-X).
In aromatic chemistry CCBFR revolve around:
With aliphatic acyclic and cyclic systems – the process is not always as straightforward – need to consider a greater array of CCBFR’s and FGI’s.
Retrosynthesis In An Aliphatic Molecule – A Guide To Alternative Disconnections.
We shall discuss possible synthesis later, but we will concentrate on CCBFR in aliphatic systems.
Review and extend CCBFR from 1C1Y, in particular:
Aldol and Claisen condensations
Alkylation of b-keto esters (RCHOCH2CO2R’)
Grignard reactions
And illustrate their use in synthesis.
There are several ways of doing this. We shall consider the following:
a) Carbanion Alkylation
i) Alkylation of enolate ions
ii) Alkylation of acetylide or cyanide
iii) Organometallic alkylation
Note
iv) Direct alkylation
of carbanions is possible in some cases
(Not a typical substitution mechanism!)
b) Carbonyl Addition And Carbonyl Substitution Reactions
i) Aldol and related reactions (Addn)
ii) Claisen condensation and related reactions (Subn)
iii) Organometallic reactions (Addn)
iv) Acetylide/cyanide addition
v) Wittig reaction (Addn)
c) Conjugate Addition Reactions - ‘Michael’ (1,4 Addition)
d) Reaction Of Alkenes, Alkynes And Aromatics
i) Pericyclic reactions:
Cycloadditions
Electrocyclic reactions
Sigmatropic reactions
ii) Friedel Crafts and related reactions
iii) Addition of carbenes to alkenes
In the main we will be looking at ionic reactions.
In CCBFR the carbonyl group is very important
Also in CCBFR, organometallic compounds are important.
e.g.
Carbonyl compounds having an a-hydrogen act as weak (protic) acids and react with a base to yield enolate anions.
Presence of neighbouring carbonyl group increases the acidity of a ketone over an alkane by a factor of 1040!
The use of such enolate anions from carbonyl compounds is fundamental to organic synthesis and you will already have met them as intermediates in the Aldol reaction and Claisen condensation.
When we have two carbonyl groups adjacent to a methylene group, the acidity of the a-H is greatly increased. Because of the acidity of their methylene (CH2) hydrogens, malonic esters, ethylacetoacetate and b-dicarbonyl compounds etc are often called active hydrogen compounds.
1) They are readily made and cheap
2) The anion can be generated quantitatively
3) Self condensation does not occur with 1 mole of base – OH is deprotonated
4) The site of deprotonation is unambiguous
5) The enolate ions formed on deprotonation can be alkylated and acylated offering useful products.
Example:
Reactions of Active Methylene Compounds
1) Carbanion Alkylation
Most important use is for preparation of ketones (from b-keto esters RCOCH2CO2Et) and of acids from malonic esters (CH2(CO2R)2).
Note:
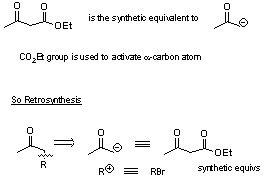
So Retrosynthesis:
Note: FGI’s can be carried out on intermediates/products.
Note especially:
Helps in the synthesis of 1,3 diols.
e.g.
2. Reaction of Active Methylene Compounds with Carbonyl Compounds (Knoevenagel Condensation)
Usually uses weak base/weak acid as catalyst, (R2NH/HOAc). Any combination of stabilising groups can be used (CN, CO2Et etc).
Carbanions derived from active methylene compounds react with a,b-unsaturated compounds by 1,4-(conjugate) addition known as Michael addition.
We have discussed the regioselective reactions of this active methylene carbon (C-2) in ethylacetoacetate. Can regiospecifically trap C-4 via the dianion.
Carbonyl Addition and Carbonyl Substitution – Aldol and Claisen Reactions.
Usually self-condensations, these reactions combine nucleophilic attack and a-substitution as the first step.
Note the Aldol condensation can also be performed with acid catalysis in which dehydration usually follows (enol form is involved – mechanism p 773). NB dehydration drives the reaction when the equilibrium is unfavourable.
Note: the only difference between the Aldol and Claisen reaction is the fate of the tetrahedral intermediate – Claisen expels alkoxide, Aldol alkoxide is protonated.
These are not very useful generally as there are four potential products. However, they can be useful if one component has no a-H.
Mixed Aldol
Only successful when one of the ester components has no a-H e.g. PhCO2Et OR HCOOEt.
Intramolecular Aldol Reactions and Claisen Condensations
When certain dicarbonyl compounds are treated with base intramolecular
Aldol reactions can occur. Similarly diesters can undergo intramolecular Claisen
Condensations (this reactions is known as the Dieckmann cyclisation).
Aldol
The intramolecular Aldol condensation forms the basis of a very useful method for making rings – The Robinson Annulation Reaction:
Reaction works best with 1,6 or 1,7 diesters to give 5 or 6 membered rings.
Regioselective Formation of Enolate Ions (p786)
Very useful method for alkene synthesis as the position of the double bond is known. The first step is formation of a Phosphorus Ylide (a neutral compound with C- and P+).
Acyl anion equivalents which exhibit Umpolung (reversed polarity p 907). Two S atoms attached to the same carbon atom of a 1,3-dithiane cause the H atoms to be more acidic (pKa about 32) than normal alkyl C-H.
1,3-dithianes are easily prepared from aldehydes, they are thioacetals.
Similar to ester dimerisation, used traditionally to make large rings.
Now improved by addition of Me3SiCl which traps the intermediate dianion.
So to finish - cis (Z) jasmone (Can. J. Chem. 1978, Vol 56, p2301)